by Marilyn Towns and Joan Bathon, M.D.
Website Director’s note: Dr. Joan Bathon founded and directed the Johns Hopkins Arthritis Center and the Arthritis Website from 1999-2010. She is now affiliated with Columbia University.
- The Interleukin-1 Gene Family
- Human Recombinant IL-1ra
- Human Clinical Trials
- Gene Therapy Approach
- Combination Study – Animal Model
- Soluble Receptor Therapy
- Potential Side Effects
- Approved Indication and Contraindication for Kineret™
- Conclusion
- References
Rheumatoid arthritis (RA) is a chronic, autoimmune, inflammatory systemic disease of unknown etiology characterized by persistent joint inflammation that results in progressive joint destruction, joint deformity, and physical disability.1 RA may affect other organs and may also result in an increased risk for premature death. The average life expectancy of RA patients is decreased by 3 to 18 years compared to age and gender matched controls.2 Damage to the bone and cartilage caused by intense episodic synovitis in RA can be attributed to various potent proinflammatory mediators known as cytokines that include interleukin-1 (IL-1), tumor necrosis factor-a(TNF-a), as well as several other cytokines (illustrated below).
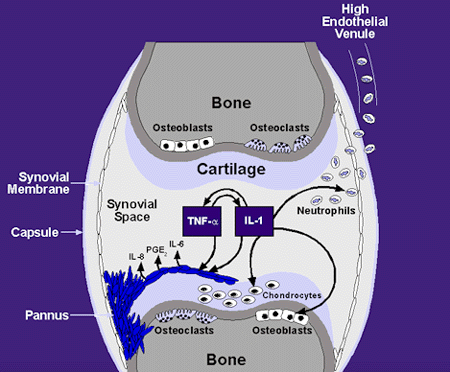
Notably, IL-1 and TNF-aare particularly abundant in the cytokine profile of the synovial lining of the joint. As a result of its potential effects on mediating joint damage, IL-1 is of particular interest in the pathogenesis of rheumatoid arthritis.
IL-1 is a cytokine that has immune and pro-inflammatory actions (slide 5) and has the ability to regulate its own expression by autoinduction.
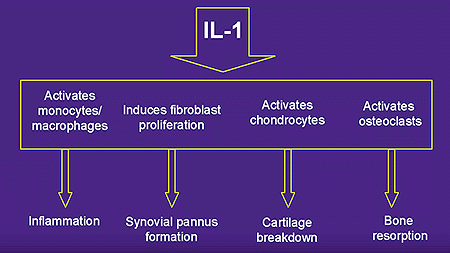
Evidence supports the fact that the level of disease activity in RA, and progression of joint destruction, correlates with plasma and synovial fluid levels of IL-1.3 IL-1 stimulates prostaglandin E2, nitric oxide, and matrix metalloproteases, which promote joint degradation. In addition, IL-1 suppresses joint repair by inhibiting collagen synthesis. Furthermore, IL-1 is an endogenous pyrogen, regulates the immune system systemically and locally in acute and chronic disease, augments activation of T and B lymphocytes, causes macrophages to release proteolytic enzymes and chemotactic factors, and also stimulates osteoclasts to resorb bone.4 The expression of the IL-1 gene may be stimulated by various types of cell interactions, by proinflammatory cytokines such as TNF-a, or by the autocrine or paracrine action of IL-1 itself.5 Similar to TNF-a, the principal cell type that produces IL-1 is the activated macrophage.
The Interleukin-1 Gene Family
The interleukin gene family is comprised of IL-1a, IL-1b, and IL-1 receptor antagonist (IL-1ra).6 IL-1aand IL-1bare proinflammatory agonist molecules, initially available as 31kD precursor molecules (pro-IL-1aand pro-IL-1b). Pro- IL-1ais biologically active while pro- IL-1brequires enzymatic cleavage to its biologically active 17kD form. IL-1ais primarily bound to the membrane whereas IL-1bis the predominant extracellular form of IL-1.
There are two specific immunoglobulin-like membrane bound IL-1 receptors (IL-1R), types I and II, found in both humans and animals. IL-1R type I is expressed constitutively at low concentration in a variety of cell types including T cells, endothelial cells, and fibroblasts. IL-1R type I has a long cytoplasmic tail and is capable of intracellular signaling.7 In contrast, IL-1R type II is expressed primarily on B cells, monocytes, and neutrophils, has a short cytoplasmic tail, and is not functionally active. Both receptors bind IL-1b with similar affinities. However, IL-1 type II receptor has a 10 to 100 fold lower binding affinity for IL-1athan IL-1R type I.8 A complete biological response requires only 1% to 2% occupancy of IL-1 type I receptors on a target cell. Soluble forms of both types of receptors are produced naturally and can compete with cell surface receptors for circulating IL-1aand IL-1b. Soluble IL-1 receptors consequently can reduce activation of cells via membrane-bound receptors.1
The third member of the interleukin gene family is IL-1ra.

IL-1ra is an endogenous receptor antagonist that exists in two forms, an intracellular (icIL-1ra)9, 10 and a secreted (sIL-1ra) form. sIL-1ra is secreted by activated monocytes and macrophages.10 The circulation of sIL-1ra is likely maintained by constitutive secretion from hepatocytes and its concentration is increased during the acute phase response.11 In contrast, icIL-1ra is retained in intact cells and is abundant in epithelial cells 9 although icIL-1ra mRNA expression can be induced in monocytes.10
Similar to IL-1, IL1-ra is primarily produced by activated monocytes and tissue macrophages, but it is also produced by a variety of other cell types. IL-1ra binds both type I and type II IL-1 receptors. The specific function of IL-1ra is to bind and block membrane-bound IL-1 receptors, thereby preventing binding and signal transduction by IL-1aand IL-1b(illustrated below).
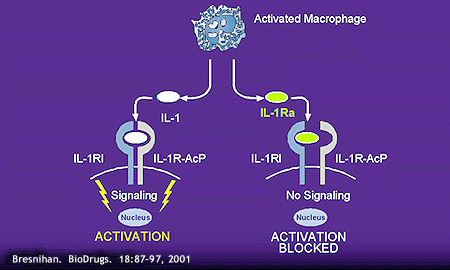
Although IL-1ra shares 30% and 19% amino acid sequence homology with IL-1aand IL-1brespectively, it does not induce signal transduction upon binding to membrane receptors.12 IL-1 R type I binds IL-1ra more avidly than IL-1, while IL-1R type II binds IL-1 more avidly than IL-1ra.13
IL-1ra levels are abundant in RA synovial tissue, however the levels are significantly less than that of IL-1. It has been demonstrated that the ratio range of IL-1ra to IL-1 of 1.2 to 3.6 in synovial tissue is considerably less than the 10 to 100-fold excess in IL1-ra necessary to inhibit 50% of the biological activity of IL-1.14-16 Since only about 5% of IL-1 receptors need to be occupied to induce cell activation, a large excess of IL-1ra would be needed therapeutically to effectively block IL-1 induced inflammation. Evidence supporting the anti-inflammatory role of IL-1ra in vivo is demonstrated by the observation that IL-1ra deficient mice spontaneously develop autoimmune diseases similar to rheumatoid arthritis and arteritis.17, 18 Hence, IL-1ra is a rational therapeutic target for use in the treatment of RA.
Human Recombinant IL-1ra
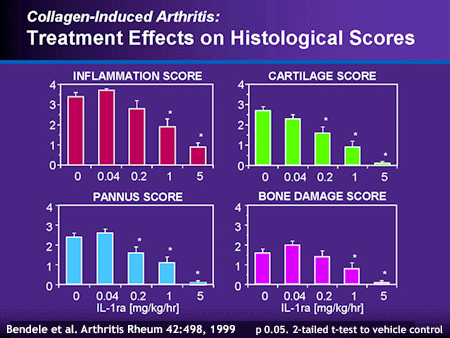
A recombinant form of human IL-1ra was developed and initially tested in animal models for arthritis. Recombinant HuIL-1ra (rHuIL-1ra) differs from the native nonglycosylated IL-1ra by the addition of an N-terminal methionine. [This form of rHuIL-1ra is also known as anakinra or Kineret™.] It binds to IL-1R type I with the same affinity as IL-1b. Administration of rHuIL-1ra to rabbits suppressed synovitis and proteoglycan loss induced by intraarticular injection of recombinant IL-1b.19, 20 In addition, inflammation and joint destruction in mice with collagen- induced arthritis was reduced by treatment with rHuIL-1ra (data illustrated below).21
An analysis by Schwab et al22 of the reactivation phase of erosive arthritis in a model of bacterial cell wall induced arthritis in rats displayed similar results. Joint swelling was reduced by at least 60% when rHuIL-1ra treatment was initiated at the onset of the reactivation phase. However, IL-1ra was ineffective if initiation of treatment was delayed by 12 to 24 hours following onset of reactivation. These encouraging results in animal models of arthritis led to trials of rHuIL-ra in human subjects with RA.
Human Clinical Trials
The first human clinical trial of rHuIL-1ra (anakinra; Kineret™) was a dose ranging, dose frequency study for safety evaluation.23 175 subjects with active RA were randomized into nine treatment groups in which placebo, 20mg, 70mg, or 200 mg rHuIL-1ra was administered subcutaneously once, 3 times or 7 times per week for three weeks, followed by four weeks of once weekly injections at the assigned doses. Generally, rHuIL-1ra was well tolerated. The most frequent adverse event was injection site reactions occurring in 67% of subjects. 41% of the 20 mg treatment group, and 73% and 77% of the 70 and 200 mg treatment groups, respectively, developed injection site reactions regardless of the frequency of dosing. In most cases the reaction was mild, localized erythema at the injection site. 5% of the subjects withdrew from the clinical trial due to this side effect. Daily dosing appeared to be more efficacious than 3 times weekly or weekly dosing for clinical parameters such as swollen joint count, and patient and investigator global assessment of disease activity. The advantage of daily dosing was further supported by the deterioration of clinical and laboratory variables, most notably CRP, when switching from a daily to weekly dosing during the second phase of the study.
Infections were less common adverse events with no apparent relationship to dosing frequency. No infections were reported in the subjects receiving the 200mg dose. Although not placebo-controlled, this open-label study provided the rationale for daily dosing of rHuIL1ra in the trials that followed and provided encouraging short-term data on the safety of this biological agent.
In a double-blind, placebo-controlled trial, Bresnihan et al24 evaluated the efficacy and safety of self-administered daily subcutaneous rHuIL-1ra at four doses. 472 patients with severe RA with randomized to one of four treatment arms (placebo or 30mg/day, 75mg/day, 150mg/day or rHuIL-1ra). The primary efficacy measure was the American College of Rheumatology (ACR 20 response) composite index. A 43% ACR 20 response rate was observed at Week 24 in the 150mg/day IL-1ra, compared to 27% in the placebo group, and a clear dose response curve to rHuIL-1ra was not observed (data illustrated below).
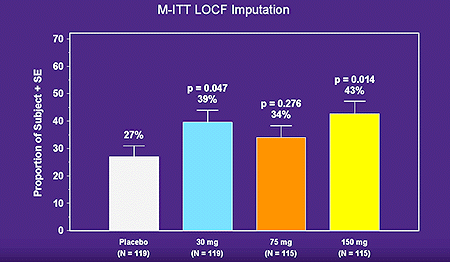
Statistically significant improvements were observed in the 150 mg/day group in individual clinical response variables, including the number of tender and swollen joints, investigator and patient assessments of disease activity, pain score, duration of morning stiffness, Health Assessment Questionnaire (HAQ) score, C-reactive protein (CRP) level, and erythrocyte sedimentation (ESR) level (data illustrated below).
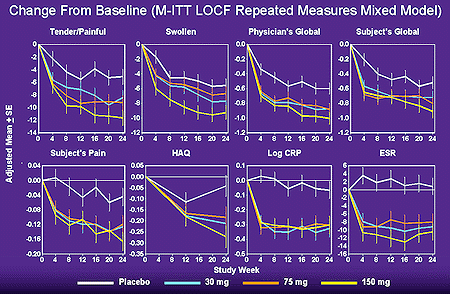
Improvements in five of the clinical response criteria were observed in both the 75 mg/day and the 50 mg/day treated groups. However, in contrast to the findings of inhibitors of tumor necrosis factor (TNF), the improvements were relatively modest, perhaps due to the short half-like (6 hrs) of rHuILra.
Generally, IL-1ra was well tolerated. The most common adverse event was injection site reaction, occurring in 25%, 50%, 73%, and 81% of patients in the placebo, 30mg, 75mg, and 150mg treatment groups, respectively. Five percent (5%) of patients receiving 150 mg/day of IL-1ra withdrew due to injection site reaction. Rates of infection were similar in the rHuIL-1ra and placebo groups. Neutropenia was observed in 3 patients receiving rHu-IL1ra and this resolved spontaneously with discontinuation of the drug. One patient in each of the rHu-IL1ra treatment groups (and none in the placebo group) developed anti-IL1-ra antibodies.
309 of the 472 subjects in the placebo-controlled trial entered into a six-month extension study (data not yet published but available on FDA web site). 76 patients who previously received placebo were randomized into one of three treatment groups (30, 75, or 150 mg rHuIL1-ra) during the 2nd six months, and displayed rates of clinical responses similar to those observed in the first 6-month phase of the study. Patients who received rHuIL1-ra in the first six months continued the same dose of drug and generally maintained their levels of clinical response during the second six months.
Bilateral poster anterior radiographs of the hands and wrists were obtained at baseline, week 24, and week 48. The rate of radiologic progression was assessed with two radiographic scoring methods, the Genant and Larsen scores. The Genant scoring method assigns separate erosion and joint space narrowing (JSN) scores as well as a combination score. The Larsen score is a single number that incorporates both erosion and joint space changes and is generally a quicker, but less sensitive, scoring method. At 24 weeks a statistically significant decrease in rate of progression of joint space narrowing (JSN) and overall total score was observed in the combined IL-1ra groups, compared to placebo, by the Genant scoring method.24, 25 The treatment effect for the combined IL-1ra groups was a 38% reduction in rate of progression in the erosion score, 58% reduction in JSN, and 47% reduction in the total Genant score, compared to the placebo group. By the Larsen scoring system, the combined IL-1ra treatment groups exhibited a 45% reduction in the total score at 24 weeks, compared to placebo. These important results confirmed the ability of IL1-ra to alter the natural history of RA since joint damage (assessed radiographically) is the hallmark of disease progression in RA and a surrogate marker for disability.
In another double blind, placebo-controlled study26, the efficacy and safety of IL1-ra added to background methotrexate (MTX) was evaluated. 419 points with moderate to severe active RA despite treatment with MTX (mean dose 16-17 mg/wk) were randomized to placebo or IL-Ira (0.04, 0.1,0.4,1.0 or 2.0 mg/kg) administered daily by subcutaneous injection. The ACR 20 response rates in the 1.0 and 2.0 mg/kg groups (46% and 38%) were statistically significantly greater than the placebo group (19%). Similar results were observed at 24 weeks. Generally, IL-1ra was safe and well tolerated. Injection site reactions occurred in 28% of placebo subjects, and in 19%, 38%, 56%, 64%, and 63% of subjects in the IL-1ra 0.04, 0.1, 0.4, 1.0 and 2.0 mg/kg groups, respectively. Injection site reactions led to early withdrawal of 7% and 10% of subjects at the 1.0 mg/kg and 2.0 mg/kg groups, respectively. This study indicated that IL1ra can be added safely to background MTX and may confer additional efficacy at the higher doses.
A long-term safety trial of huIL-1ra (anakinra) is currently in progress. 1414 subjects with RA who were receiving a variety of concurrent disease-modifying agents (DMARDs) other than the TNF inhibitors, Enbrel® and Remicade®, were randomized to receive IL-1ra (100 mg/day) or placebo. Patients with a predisposition to infection were not excluded. Preliminary results at six months27 indicated a modest increase in incidence of serious infection in the anakinra group relative to placebo (approximately 2 vs. 1%, respectively). The long-term data from this study are expected at a later date.
Gene Therapy Approach
A limitation to the successful use of IL-1ra as a treatment for RA is its short (six hours) half-life in plasma.23 Daily injections are required to sustain a therapeutic effect. Additionally, a large excess of IL-1ra is required to block the effect of IL-1. Eventually, the use of gene therapy may be a more efficient method to provide sustained high local concentrations of IL-1ra. Feasibility studies have been performed in which synovial fibroblasts were transfected with the gene for human IL-1ra and subsequently injected into the joints of animals28. IL-1ra was successfully produced but expression tapered off after several weeks28. In humans, the IL-1ra gene was introduced into the joints of three patients prior to arthroplasty (joint replacement) in the hands. Analysis of the joint tissue following arthroplosty demonstrated successful induction of IL-1ra expression29. Methods for sustaining long-term expression of genetically induced proteins are still in their infancy, however.
Combination Study – Animal Model
IL1 and TNF have very similar biological actions and act synergistically to promote inflammation in vitro. Consequently, a therapeutic approach in which inhibitors of both IL1 and TNF-a are combined could represent a very potent treatment for RA. Bendele et al30 explored this approach in mice with collagen induced arthritis (CIA) and rats with adjacent induced arthritis (AIA). Various doses of IL-1ra (20 or 100mg/kg) in slow release hyaluronic acid and PEGylated soluble TNF receptor type I (PEG sTNFRI) (0.3, 1, 3mg/kg) were given alone or in combination. Treatment effects were measured by sequential caliper measurements of the ankle joints; paw volume, final paw weight, and evaluation of bone and cartilage legions. The 20-mg/kg dose of IL-1ra provided no benefit alone, whereas a dose related effect was observed with sTNFR alone. However, in combination with any dose of sTNFRI, the 20mg/kg dose of IL-1ra displayed efficacy.
A preliminary open-label safety study in a small number of patients with RA treated with the combination of etanercept (sTNF-RII) and sHuIL-1ra was reported recently31. The observed incidence of serious infections was high however (7 %) and may be a dose-limiting problem with this therapeutic approach. A double-blind controlled trial is now underway in a larger number of patients.
Soluble Receptor Therapy
Another biological strategy for inhibiting IL1 is via the use of soluble recombinant human IL-1 receptors. Drevlow et al32 evaluated the efficacy of soluble IL-1 receptor type 1 (sIL1R type I) and observed only a marginal clinical benefit at the highest dose. In fact, exacerbation of disease was observed at the lower dosing levels. This may be because sIL-1R type I binds IL-1ra more avidly than IL1 and may therefore have a proinflammatory, rather than anti inflammatory, effect. Clinical studies with sIL1 R type II are now in progress in RA. In rabbit antigen induced arthritis, sIL1-R type II significantly inhibited soft tissue swelling and joint damage.33
Potential Side Effects
Injection Site Reactions
The most commonly observed side effect in all of the clinical trials with HuIL-1ra (anakinra) to date is injection site reactions, occurring in approximately two-thirds of patients at the FDA-approved daily dose of 100 mg administered subcutaneously. These reactions are generally mild, present as erythema, itchy and discomfort and resolve over one to two months.
Infections and Neutropenia
A modest increase in the risk of serious infection was observed in RA patients treated with anakinra in combination with DMARDS other than TNF inhibitors, compared to placebo with DMARDs (2 % vs 1%). The risk of serious infections of anakinra in combination with a TNF inhibitor is unknown, but preliminary data in a small-uncontrolled study of 58 RA patients suggest an increase (7%) relative to anakinra alone.
In the placebo-controlled trials, 8% of patients receiving anakinra had mild to moderate decreases in absolute neutrophil counts, compared to 2% in the placebo groups. 0.3% of anakinra treated patients experiences severe neutropenia (< 1x 109/L). In the combination anakinra/etanercept (Kineret™/Enbrel®) study, two of 58 points (3%) experienced severe neutropenia.
Malignancy
The rate of malignancies in HuIL-1ra treated patients was not increased relative to expected rates in the general population.
Immunogenecity
28% of anakinra-treated patients tested positively for anti-anakinra antibodies in a very sensitive assay. However, neutralizing antibodies were rare, and the presence of antibodies did not appear to correlate with clinical response or adverse events.
Approved Indication and Contraindication for Kineret™
Kineret™ [Anakinra; hu SIL1ra] was recently approved by the FDA for the reduction in signs and symptoms of moderately to severely active RA in patients 18 or older who have failed 1 or more DMARDS. Kineret™ can be used alone or in combination with DMARDs other than TNF blocking agents (Enbrel®; Remicade®). The approved dosage is 100mg subcutaneously once daily. Kinert™ is not recommended for use in combination with TNF inhibitors.
Kineret™ should be discontinued in patients who develop serious infections and should not be initiated in patients with active infections. The safety and efficacy of combined therapy with IL-2 and TNF inhibitors has yet to be defined. Until then, the FDA recommends the use of Kineret™ and TNF inhibitors (Enbrel®; Remicade®) only if no satisfactory alternatives exist.
Conclusion
Evidence in animal and human studies supports the central role of IL-1 in the pathogenesis of RA. Therefore, inhibition of the proinflammatory actions of IL-1 is paramount in preventing the mechanisms of initiation in RA, which ultimately result in the destructive properties of inflammation.
The inhibition of a single cytokine in the treatment of rheumatoid arthritis is problematic as various cytokines have synergisitic biological activities. For example, TNF-ais important in inflammation and tissue destruction, upregulates the production of IL-1 through autocrine and paracrine mechanisms and hepatic synthesis of IL-6.32 Both IL-1 and TNFatrigger activation and proliferation of synovial cells, induce collagen synthesis, inhibit proteoglycan synthesis, and stimulate bone resorption, induce production of other cytokines, and upregulate the expression of adhesion molecules on endothelial cells.
Therefore, it would be imperative to find an innovative therapeutic treatment using combination therapy for use in RA. Presently, ongoing studies combining the use of an IL-1ra inhibitor with a TNF-ainhibitor may be the most efficacious method to treat RA. TNF inhibitors control inflammation significantly and IL-1ra appears to suppress bone resorption and osteopenia. The pleiotropic effects of both IL-1 and TNF-asuggest there is a cascade release of cytokines. Thus, modulation of the specific cytokine triggering the cascade may be more beneficial to developing an efficacious and synergistic treatment of RA. However, the inhibition of proximal cytokines IL-1 and TNF-ais the primary focus at present. The two approaches represent a novel complementary strategy for the treatment of RA. Current studies using IL-1ra or soluble receptor IL-1R type II alone and in combination with TNF inhibitors are ongoing. Finally, the development of a drug delivery system permitting IL-1ra to block the receptor for a greater period of time while simultaneously maintaining concentration of antagonists would be optimal for efficacy in either a monotherapy or combination therapy for therapeutic treatment in RA.
Addendum and update:
Other inhibitors of IL-1 have been tested in RA including monoclonal antibodies against the cytokine and a fusion construct of IL1ra and the IL1r accessory protein with Fc immunoglobulin (rilonacept) and an IL1 converting enzyme inhibitor (pralnacasan). These studies did not demonstrate a robust effect of IL-1 inhibition in RA. However some of these agents have been demonstrated to be very effective in the treatment of pyrin-associated febrile syndromes (Muckle Wells, NOMID, etc) and are now approved for these rare conditions.
References
- Choy, EHS, Panayi,GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. NEJM 344:907-916, 2001.
- Pincus T, Callahan LF. Taking mortality in rheumatoid arthritis seriously-predictive markers, socioeconomic status and comorbidity. J Rheumatol 13:841-845, 1986.
- Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma Interleukin-1 levels with disease activity in rheumatoid arthritis. Lancet 2:706-709, 1998.
- Bresnihan, B., Cunane, G. Interleukin-1 Receptor Antagonist. Emerging Therapies for Rheumatoid Arthritis. Rheum Dis Clin North Am 24:615-628, 1998.
- Albani S, Carson DA. Etiology and pathogenesis of rheumatoid arthritis. Arthritis and Allied Conditions I: 979-992, 1997.
- Arend WP: Interleukin-1 receptor antagonist: A new member of the interleukin 1 family. J Clin Invest 88:1445-1451, 1991.
- Sims JE, Gayle MA, Slack JL, et al. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA 90:6155-6159, 1993.
- Dower SK, Wignall J, Schooley K, McMahan CJ , Jackson J., Prickett KS, Lupton S, Cosman, D, and Sims JE. J Immunol 142:4312-4320, 1989.
- Haskill, S., Martin, G., van Le, L., Morris, J. , Peace, A., Bigler, C.F., Jaffe, G.J., Hammerber, C., Sporn, S.A., Fong, S. et al. cDNA cloning of an intracellular form of the human interleukin-1 receptor antagonist associated with epithelium. Proc Natl Sci USA 88:3681-3685, 1991.
- Gabay, C., Porter, B., Fantuzzi, G., Arend, W.P. Mouse Il-1 receptor antagonist isioforms: complementary DNA cloning and protein expression of intracellular isiform and tissue distribution of secreted and intracellular IL-1 receptor antagonist in vivo. J Immunol 159:5905-5913, 1997.
- Gabay, C., Smith, M.F., Eidlen, D., Arend, W.P. Interleukin 1 receptor antagonist (IL1RA) is an acutephase protein. J Clin Invest 99:2930-2940, 1997.
- Dripps, D.J., B.J. Brandhuber, R.C. Thompson, and S.P. Eisenberg. Interleukin-1 receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem 266:10331-10336, 1991.
- Arend WP, Welgus, HG, Thompson,RC, Eisenberg SP, Biological Properties of Recombinant Human Monocyte-Derived Interleukin-1 Receptor Antagonist. J Clin Invest 85:1694-1697, 1990.
- Horai R, Saijo S, Tanoika H, et al. Development of chronic arthropathy resembling rheumatoid arthritis in interleukin-1 receptor antagonist-deficient mice. J Exp Med 191:313-320, 2000.
- Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin-1 receptor antagonist gene. J Exp Med 191:303-312, 2000.
- Smith RJ, Chin JE, Sam LM, Justen JM. Biologic Effects of an Interleukin-1 Receptor Antagonist Protein On Interleukin-1 Stimulated Cartilage Erosion and Chondrocyte Responsiveness. Arthritis Rheum 34:78-83, 1991.
- Seckinger P, Klein-Nulend J, Alander C, Thompson RC, Dayer JM, Raisz LG. Natural Recombinant Human IL-1 Receptor Antagonist Block the Effects of IL-1 on Bone Resorption and Prostaglandin Production. J Immunol 145:4181-4184, 1990.
- Bendele A, McAbee T, Sennello G, Frazier J, Chlipala E, McCabe D. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: Comparison of efficacy in animal models with human clinical data. Arthritis Rheum 42(3):498-506, 1999.
- Schwab JH, Anderle SK, Brown RR, Dalldorf FG, Thompson RC, Pro-and anti-inflammatory froles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis. Infect Immun 12:4436-4442, 1991.
- Campion, G.V., Lebsack, M.E., Lookabaugh, J., Gordon, G., Catalnao, M., The IL-1RA Study Group. Dose Range and Dose Frequency Study of Recombinant Human Interleukin-1 Receptor Antagonist in Patients with Rheumatoid Arthritis. Arthritis Rheum 39:1092-1101, 1996.
- Bresnihan B, Alvaro-Garcia JM, Cobby M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, Watt I, Williams B, Aitchinson R, McCabe D, Musikic P. Treatment of rheumatoid arthritis with recombinant human interleukin receptor antagonist. Arthritis Rheum 41(12): 2196-2204, 1998.
- Jiang, Y., Genent H.K., Watt, I., Cobby, M., Breshinan, B., Aitchison, R., McCabe D. A Multicenter, Double Blind, Dose-Ranging, Randomized, Placebo-Controlled Study of Recombinant Human Interleukin-1 Receptor Antagonist In Patients with Rheumatoid Arthritis. Arthritis Rheum 43:1001-1009, 2000.
- Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland L, Kremer J, Bear MB, Rich WJ, McCabe D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46(3):614-624, 2002.
- Fleischman R, Tesser J, Schechtman J, Modafferi D, Poulakos J, Bennett R, Burmester G-R, Handel M, Sun G. A safety trial of anakinra: Recombinant interleukin-1 receptor antagonist (IL-Ra) in a large, placebo controlled heterogeneous population of patients with rheumatoid arthritis. Arthritis Rheum 44(9 suppl):S84, 2001.
- Makarov SS, Olsen JC, Johnston WN, et al. Suppression of experimental arthritis by gene transfer of interleukin-1 receptor antagonist cDNA. Proc Natl Acad Sci USA 1996; 93:402-406.
- Ghivizzani SC, Kang R, Muzzonigro T, et al. Gene therapy for arthritis treatment of the first three patients. Arthritis Rheum 40:suppl:S223-S223, 1997.
- Bendele A, Chlipala ES, Scherrer J, Frazier J, Sennello G, Rich W, Edwards CK. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum 43:2648-2659, 2000.
- Zhu L, Bolon B, Hu, Y-L, Feige U, Zack D. Efficacy of treatment with anakinra in combination with other therapeutics in rat models of arthritis. Arthritis Rheum 44(9 suppl):S241, 2001.
- Drevlow B, Lovis R, Haag MA, Sincacore JM, Jacobs J, Blosche C, Landay A, Moreland LW, Pope RM. Recombinant human interleukin-1 receptor type I in the treatment of patients with active rheumatoid arthritis. Arthritis Rheum 39:257-265, 1996.
- Dawson J, Englehardt P, Kastelic T, Cheneval D, Mackenzie A, Ramage P. Effects of soluble interleukin-1 type II receptor on rabbit antigen-induced arthritis: clinical, biochemical, and histological assessment. Rheumatology 38:401-406, 1999.

