[…]
[…]
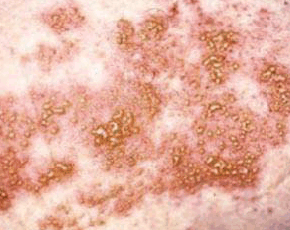
Type II collagen is abundant in the joint and is a major target of immune-mediated damage in RA. As suggested in studies of experimental animals, induction of immune tolerance at the level of the cartilage may be a way of reducing the signs and symptoms of RA and prevent joint destruction. However, whether oral administration of Type II collagen is an efficacious treatment for humans with established active RA has not been established.
Increasing the dose of infliximab and, in some cases, adalimumab can result in improved efficacy in RA patients with inadequate responses to initial dosing. However, whether increasing the dose of etanercept can also result in clinical improvement has not been studied.
The U.S. Food and Drug Administration released an early communication regarding a plan to investigate a possible association between TNF inhibitor therapy and cancer in children receiving these agents for treatment of juvenile idiopathic arthritis (JIA) and Crohn’s disease.
The T-cell inhibitor efalizumab (Raptiva) has demonstrated efficacy in the treatment of moderate to severe plaque psoriasis. However, it has not demonstrated efficacy for the treatment of psoriatic arthritis and, in one study, was associated with worsening of arthritis symptoms. Only a minority of individuals with psoriasis have a concomitant inflammatory arthritis, making therapy with efalizumab an option for the treatment of a large number of psoriasis patients who have no articular involvement.