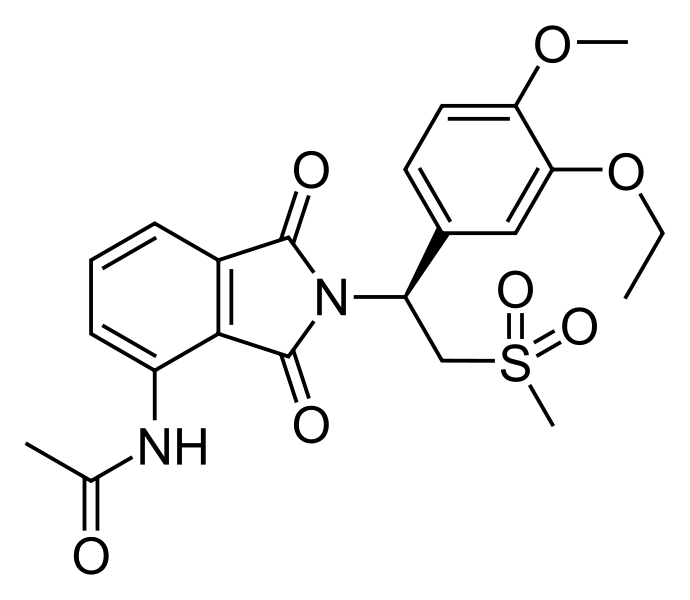
Apremilast (Otezla®) received FDA approval on March 21, 2014 for the treatment of adults with active psoriatic arthritis (PsA). It is the first oral medication in the U.S. with an approved indication for the treatment of PsA. The recommended dose is 30 mg BID