[…]
The efficacy of rituximab, a monoclonal antibody directed against CD20 positive B cells, has been demonstrated for the treatment of rheumatoid arthritis (RA) in a number of key studies. However, not all patients respond to therapy. Whether patients with initial non-response may benefit from follow-up treatments (i.e. initial treatment resistance) has not been investigated.
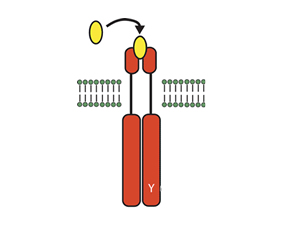
Intracellular signaling mechanisms relay cell signals from membrane bound receptors to the nucleus, where transcription of DNA initiates an effector response. In inflammatory cells, the effector response helps to drive the initiation and propagation of inflammatory diseases, such as rheumatoid arthritis (RA). Thus, inhibiting key intracellular signals may be a treatment for RA.
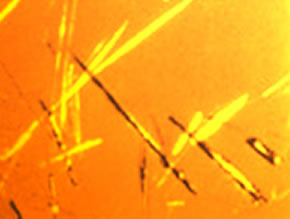
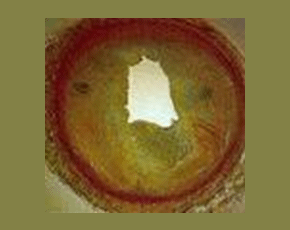
Urate lowering therapy can be very effective for reducing flares of gout, thereby preventing ongoing joint damage and deformity. Despite this efficacy, most gout patients are undertreated, leading to undue painful flares and joint damage. Non-adherence to therapy is a strong contributor to undertreatment.
Glucosamine, with or without chondroitin, is a popular and high selling neutraceutical with claims to improve symptoms associated with painful osteoarthritis (OA). Clinical trials testing purported benefits have yielded mixed results, with manufacturer sponsored trials showing benefits while most unbiased studies have generally not shown benefit over placebo. Previously, in a large clinical trial of glucosamine hydrocholoride with or without chondroitin sulfate, neither agent demonstrated efficacy over placebo in treating pain in individuals with knee OA.

At their November 24, 2008 meeting, the FDA Arthritis Drug Advisory Committee heard updated efficacy and safety data for febuxostat for the treatment of gout-associated hyperuricemia. The committee’s recommendation was carried on a vote of 12 to 0. Final FDA approval of the drug is pending; however, FDA approval generally follows the recommendations of the advisory committee.